How to Calculate Ph
Plug in the information into the formula. Potassium Phosphate pH 58 to 80 preparation guide and recipe.

Ph Poh H3o Oh Kw Ka Kb Pka And Pkb Basic Calculations Acids And Bases Chemistry Problems Teaching Chemistry Chemistry Lessons Chemistry Classroom
In this case we are finding pOH and pH is known so the formula is.

. The pH scale is logarithmic and inversely indicates the. NaOH dissociate completely into Na aq OH-aq ions in water. Pick one of the formulas.
If you are doing a chemistry problem look at the equation to identify the. Learn about our Editorial Process. PH - log H 3 O.
To calculate your GPA divide the total number of grade points earned by the total number of letter graded units undertaken. The Concentration of hydrogen ion given pH formula is defined as the reciprocal of ten to the power of pH is calculated using Concentration of Hydrogen Ion 10-Negative Log of Hydronium ConcentrationTo calculate Concentration of Hydrogen Ion given pH you need Negative Log of Hydronium Concentration pHWith our tool you need to enter the respective value for. The pH value is logarithmically and is inversely related to the concentration of hydrogen ions in a solution.
Helmenstine holds a PhD. We assume that after adding KOH volume of solute is not changed much. M mol dm-3.
To calculate kinetic energy write out a formula where kinetic energy is equal to 05 times mass times velocity squared. Subtract this from the Pres Rdg from your last bill. Find expected pH for a given concentration simply by entering the molarity or enter weight and total volume.
Plug in the information into the formula. PH -logH log 1H The pH scale ranges from 0 to 14 with 70 being neutral. Go through the simple and easy guidelines on how to measure pH value.
Concentration of KOH in mol dm-3 Calculate pH. The pH calculator tool provides expected pH values for a variety of common laboratory and industrial chemicals. Add in the value for the mass of the object then the velocity with which it is moving.
Calculate the pH of a 0100 M nitric acid solution. The pH power of hydrogen of a solution is a measure of the concentration of hydrogen ions and is also a measure of acidity but it isnt the same as K a. Now what is the pOH of the solution above.
Solutions with a pH below 70 are acidic while solutions with a pH above 70 are basic or alkaline. If you want to be savvy about your electric bill heres a step-by-step guide on how to compute it accurately. In biomedical sciences and is a science writer educator and consultant.
You can even calculate the impact of dilution entering starting molarity and final volume. She has taught science courses at the high school college and graduate levels. Find the pH of a 00025 M HCl solution.
Get your last electric bill. For each unit of credit the following grade points are earned. PH is the negative base 10 logarithm log on a calculator of the hydrogen ion concentration of a solution.
Nitric acid has a chemical formula of HNO 3. The HCl is a strong acid and is 100 ionized in water. If you are doing chemistry in a lab you will need to determine the concentration by finding the moles per unit of volume mv or M.
Go to your electric meter and get the current reading. PH-log02M Enter and look on the graphing calculator for the answer. It commonly ranges between 0 and 14 but can go beyond these values if sufficiently acidicbasic.
To calculate the pH of an aqueous solution you need to know the concentration of the hydronium ion in moles per liter. Recipe can be automatically scaled by entering desired final volume. In this video I will go through a worked example showing you two methods that you can use to calculate the concentration of hydroxide ions in a solution usin.
To calculate pH remember that the pH scale goes from 0 to 14 with numbers below 7 being acidic and numbers above 7 being basic. Calculate pH when amount mol of KOH is known and volume of solute is known. The pH scale pH is a numeric scale used to define how acidic or basic an aqueous solution is.
HNO 3 is another strong acid so the pH of this solution will also be less than 7. The room temperature is 25 0 C. You can also calculate the pOH and the concentration of hydroxide ions.
These buffers have excellent buffering capacity and are highly soluble in water. Discrepancies in using pOH vs pH to solve HOH- concentration change problem. We solve this example according to method 1.
In mathematical terms pH is the negative logarithm of the molar concentration of hydrogen ions in the solution. HOW TO CALCULATE YOUR ELECTRIC BILL. To calculate it take the log of a given hydrogen ion concentration and reverse the sign.
The pH to H formula that represents this relation is. A pH testing strip will tell you that NaOH sodium hydroxide is a strong alkaline but to calculate its exact pH you have to work out its molarity first. Using the 0100 M nitric acid as the H concentration of hydrogen ions the solution is as follows.
Calculate the pH by using the pH to H formula ie pH-logH. Calculate pH of 01 moldm-3 NaOH solution. Use Excel to Calculate MAD.
Potassium phosphate buffers sometimes called Gomori buffers consist of a mixture of monobasic dihydrogen phosphate and dibasic monohydrogen phosphate. Since the pH scale is logarithmic not linear a solution of pH 1 would have ten times not twice the H that a solution of pH 2. Find the Pres Rdg at the back of your bill.
The pH is then calculated using the expression. Know the concentration of hydrogen ions in the solution. Calculating pH when weak base is added to an strong acid.
How to calculate pH of basic salt solution mixed with strong base. To optimize your forecast whether moving average exponential smoothing or another form of a forecast you need to calculate and evaluate MAD MSE RMSE and MAPE. In chemistry pH p iː ˈ eɪ tʃ historically denoting potential of hydrogen or power of hydrogen is a scale used to specify the acidity or basicity of an aqueous solutionAcidic solutions solutions with higher concentrations of H ions are measured to have lower pH values than basic or alkaline solutions.
First we calculate pOH and then calculate the pH using the relationship of. With Excel 2016 or later this is easy to do. Calculate pH when concentration of KOH in mol dm-3 is known.
Theres a relationship between the two though and you can calculate K a for an acid if you know the concentration of acid and the pH of the solution. So you can enter volume of solution as volume of solute. Solve for the unknown variable.
POH 0699 14. We have to calculate concentration of OH-aq to calculate pH. Finding the pH of a mixture of weak acid and strong base.
The Mean Absolute Deviation MAD is the sum of absolute differences between the actual value and the forecast divided. Larger values signify stronger acids. A pH lower than 7 is acidic while a pH higher than 7 is alkaline.

How To Calculate The Ph Poh H Oh Of Strong Acids Youtube Ap Chemistry Biochemistry Chemistry

How To Calculate Ph If Hydrogen Ion Concentration Is Given For All Stud Student Concentration Calculator
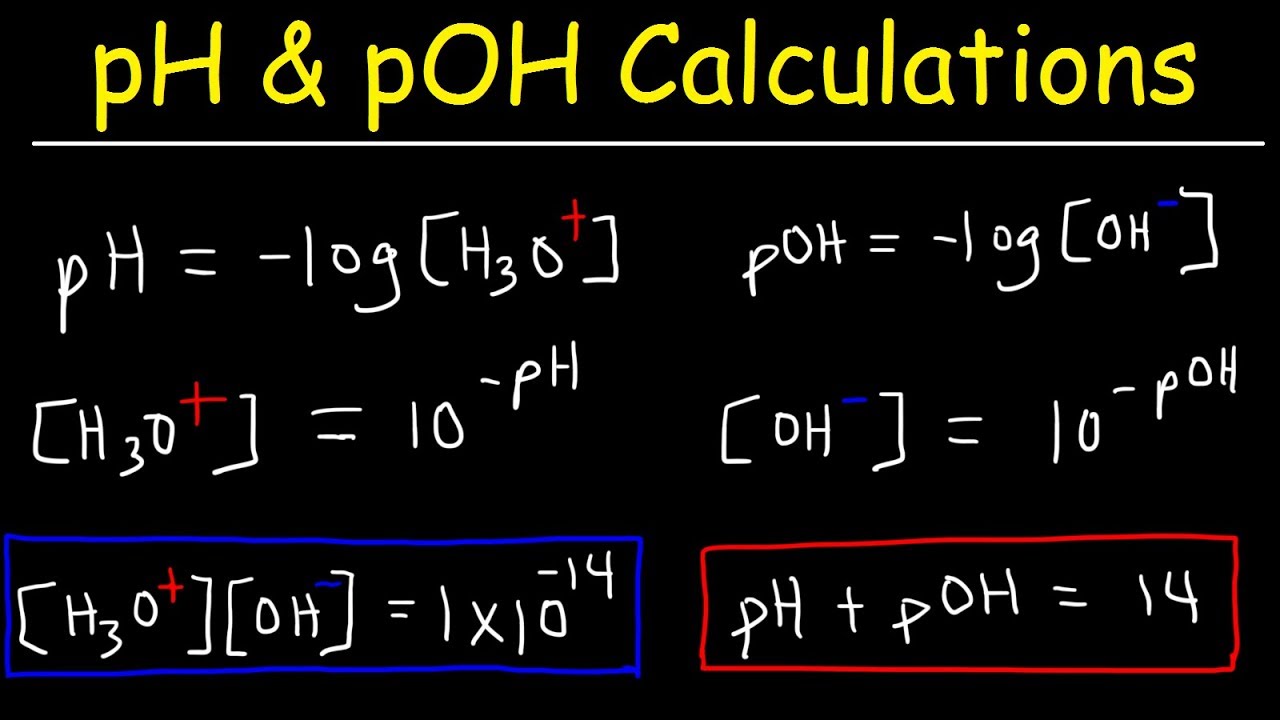
Ph Poh H3o Oh Kw Ka Kb Pka And Pkb Basic Calculations Acids And Bases Chemistry Problems Teaching Chemistry Chemistry Lessons Chemistry Classroom

No comments for "How to Calculate Ph"
Post a Comment